Difference between revisions of "Template:Infobox chromium"
Jump to navigation
Jump to search
imported>Materialscientist m (Reverted edits by 69.11.220.126 (talk) to last version by Amirobot) |
imported>Monkbot m (Task 18a (cosmetic) (manual): eval 2 templates: del empty params (2×);) |
||
| (82 intermediate revisions by 31 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Infobox element |
| + | |engvar={{{engvar|}}} | ||
|name=chromium | |name=chromium | ||
| − | |||
|number=24 | |number=24 | ||
|symbol=Cr | |symbol=Cr | ||
| + | |abundance= | ||
| + | |abundance in earth's crust= | ||
| + | |abundance in oceans= | ||
| + | |abundance in solar system= | ||
|left=[[vanadium]] | |left=[[vanadium]] | ||
|right=[[manganese]] | |right=[[manganese]] | ||
| − | |above= | + | |above=– |
|below=[[molybdenum|Mo]] | |below=[[molybdenum|Mo]] | ||
| − | | | + | |category comment= |
| − | |||
|group=6 | |group=6 | ||
|period=4 | |period=4 | ||
|block=d | |block=d | ||
| − | |||
| − | |||
|appearance=silvery metallic | |appearance=silvery metallic | ||
| − | |image name= Chromium_crystals_and_1cm3_cube.jpg | + | |image name=Chromium_crystals_and_1cm3_cube.jpg |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|electrons per shell=2, 8, 13, 1 | |electrons per shell=2, 8, 13, 1 | ||
| − | + | |phase= | |
| − | |phase= | ||
|phase comment= | |phase comment= | ||
|density gplstp= | |density gplstp= | ||
| Line 45: | Line 37: | ||
|heat fusion=21.0 | |heat fusion=21.0 | ||
|heat fusion 2= | |heat fusion 2= | ||
| − | |heat vaporization= | + | |heat vaporization=347 |
|heat capacity=23.35 | |heat capacity=23.35 | ||
|vapor pressure 1=1656 | |vapor pressure 1=1656 | ||
| Line 55: | Line 47: | ||
|vapor pressure comment= | |vapor pressure comment= | ||
|crystal structure=body-centered cubic | |crystal structure=body-centered cubic | ||
| − | |||
| − | |||
|electronegativity=1.66 | |electronegativity=1.66 | ||
|number of ionization energies=4 | |number of ionization energies=4 | ||
| − | | | + | |ionization energy 1=652.9 |
| − | | | + | |ionization energy 2=1590.6 |
| − | | | + | |ionization energy 3=2987 |
| − | |atomic radius= | + | |atomic radius=128 |
|atomic radius calculated= | |atomic radius calculated= | ||
| − | |covalent radius= | + | |covalent radius=139±5 |
|Van der Waals radius= | |Van der Waals radius= | ||
| − | |magnetic ordering=[[antiferromagnetism| | + | |magnetic ordering=[[antiferromagnetism|antiferromagnetic]] |
| + | |magnetic ordering comment=(rather: [[spin density wave|SDW]])<ref name=fawcett>{{cite journal|doi=10.1103/RevModPhys.60.209|title=Spin-density-wave antiferromagnetism in chromium|year=1988|author=Fawcett, Eric|journal=Reviews of Modern Physics|volume=60|pages=209|bibcode=1988RvMP...60..209F}}</ref> | ||
| + | |electrical resistivity unit prefix=n | ||
|electrical resistivity= | |electrical resistivity= | ||
|electrical resistivity at 0= | |electrical resistivity at 0= | ||
| − | |electrical resistivity at 20=125 | + | |electrical resistivity at 20=125 |
|thermal conductivity=93.9 | |thermal conductivity=93.9 | ||
|thermal conductivity 2= | |thermal conductivity 2= | ||
| Line 78: | Line 70: | ||
|speed of sound rod at 20=5940 | |speed of sound rod at 20=5940 | ||
|speed of sound rod at r.t.= | |speed of sound rod at r.t.= | ||
| + | |magnetic susceptibility= +280.0·10<sup>−6</sup> | ||
| + | |magnetic susceptibility ref=  (273 K)<ref>{{Cite book|title=CRC, Handbook of Chemistry and Physics|last=Weast|first=Robert|publisher=Chemical Rubber Company Publishing|year=1984|isbn=0-8493-0464-4|location=Boca Raton, Florida|pages=E110}}</ref> | ||
|Young's modulus=279 | |Young's modulus=279 | ||
|Shear modulus=115 | |Shear modulus=115 | ||
|Bulk modulus=160 | |Bulk modulus=160 | ||
|Poisson ratio=0.21 | |Poisson ratio=0.21 | ||
| + | |Vickers hardness=1060 | ||
|Mohs hardness=8.5 | |Mohs hardness=8.5 | ||
| − | + | |Brinell hardness=687–6500 | |
| − | |Brinell hardness= | ||
|CAS number=7440-47-3 | |CAS number=7440-47-3 | ||
|isotopes= | |isotopes= | ||
| − | {{ | + | {{infobox element/isotopes stable | mn=50 | sym=Cr | na=4.345% | n=26 |firstlinks=yes}} |
| − | {{ | + | {{infobox element/isotopes decay2 | mn=51 | sym=Cr | na=[[synthetic radioisotope|syn]] | hl=27.7025 d |
| − | dm2=[[gamma ray|γ]] | de2=0.320 | pn2= | ps2= | + | |dm1=[[electron capture|ε]] | de1=– | link1=vanadium-51 | pn1=51 | ps1=V |
| − | {{ | + | |dm2=[[gamma ray|γ]] | de2=0.320 | pn2= | ps2=– }} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=52 | sym=Cr | na=83.789% | n=28 |firstlinks=no}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=53 | sym=Cr | na=9.501% | n=29 |firstlinks=no}} |
| + | {{Infobox element/isotopes stable | mn=54 | sym=Cr | na=2.365% | n=30 |firstlinks=no}} | ||
|isotopes comment= | |isotopes comment= | ||
| − | }}<noinclude> | + | |discovery and first isolation by=[[Louis Nicolas Vauquelin]] |
| − | {{Template reference list}} | + | |discovery date=1794 |
| + | |first isolation date=1797 | ||
| + | |QID=Q725 | ||
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Cr}} | ||
| + | {{Template reference list}} | ||
| + | {{documentation|1=Template:Infobox element/doc}} | ||
</noinclude> | </noinclude> | ||
Latest revision as of 19:29, 16 December 2020
[[Category:Template:Pagetype with short description]]
 | |||||||||||||||||||||||||||||||||
| Chromium | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | silvery metallic | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Cr) | Template:Val[1] | ||||||||||||||||||||||||||||||||
| Chromium in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 24 | ||||||||||||||||||||||||||||||||
| Group | group 6 | ||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d5 4s1 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 13, 1 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 2180 K (1907 °C, 3465 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 2944 K (2671 °C, 4840 °F) | ||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.19 g/cm3 | ||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.3 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 21.0 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 347 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 23.35 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −4, −2, −1, 0, +1, +2, +3, +4, +5, +6 (depending on the oxidation state, an acidic, basic, or amphoteric oxide) | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.66 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128 pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 139±5 pm | ||||||||||||||||||||||||||||||||
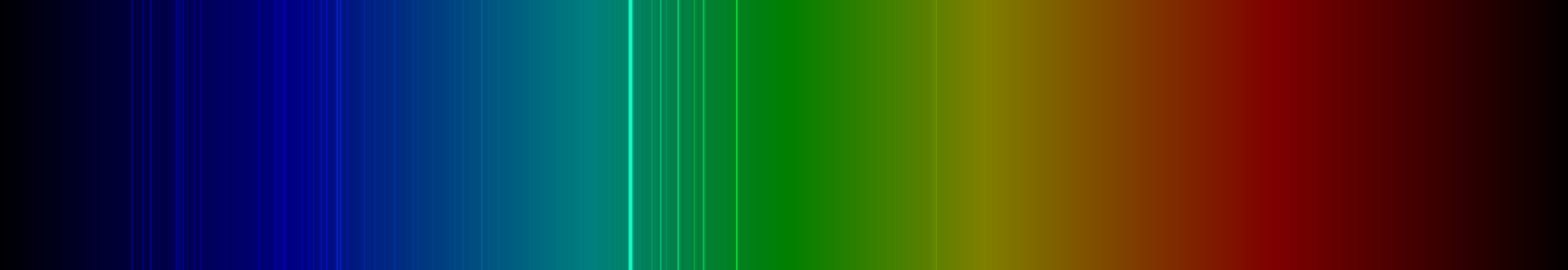
| Spectral lines of chromium | |||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
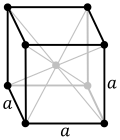
| Crystal structure | body-centered cubic (bcc) | ||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5940 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||
| Thermal expansion | 4.9 µm/(m·K) (at 25 °C) | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 93.9 W/(m·K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 125 nΩ·m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | antiferromagnetic (rather: SDW)[2] | ||||||||||||||||||||||||||||||||
| Magnetic susceptibility | +280.0·10−6 cm3/mol (273 K)[3] | ||||||||||||||||||||||||||||||||
| Young's modulus | 279 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 115 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 160 GPa | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.21 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 8.5 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 1060 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 687–6500 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7440-47-3 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Discovery and first isolation | Louis Nicolas Vauquelin (1794, 1797) | ||||||||||||||||||||||||||||||||
| Main isotopes of chromium | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]
| data m.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 1907 | — | — | ||
| K | 2180 | 2180 | 0 | ||
| F | 3465 | 3465 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 1907, K: 2180, F: 3465 | ||||
| comment | |||||
| data b.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 2671 | — | — | ||
| K | 2944 | 2944 | 0 | ||
| F | 4840 | 4840 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 2671, K: 2944, F: 4840 | ||||
| comment | |||||
| V ← |
→ Mn | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Fawcett, Eric (1988). "Spin-density-wave antiferromagnetism in chromium". Reviews of Modern Physics. 60: 209. Bibcode:1988RvMP...60..209F. doi:10.1103/RevModPhys.60.209.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
Lua error: Internal error: The interpreter has terminated with signal "24".