Difference between revisions of "Template:Infobox iron"
Jump to navigation
Jump to search
imported>Stone (changed image to a better one) |
imported>Martin Embeh m (added lattice parameter for BCC Fe from https://books.google.de/books?id=PvU-qbQJq7IC&pg=PA65#v=onepage&q&f=false (same as german wikipedia)) |
||
| (115 intermediate revisions by 48 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{infobox element |
|name=iron | |name=iron | ||
|number=26 | |number=26 | ||
|symbol=Fe | |symbol=Fe | ||
| + | |abundance= | ||
| + | |abundance in earth's crust= | ||
| + | |abundance in oceans= | ||
| + | |abundance in solar system= | ||
|left=[[manganese]] | |left=[[manganese]] | ||
|right=[[cobalt]] | |right=[[cobalt]] | ||
| − | |above= | + | |above=– |
|below=[[ruthenium|Ru]] | |below=[[ruthenium|Ru]] | ||
| − | | | + | |category comment= |
| − | |||
|group=8 | |group=8 | ||
|period=4 | |period=4 | ||
|block=d | |block=d | ||
| − | |||
| − | |||
|appearance=lustrous metallic with a grayish tinge | |appearance=lustrous metallic with a grayish tinge | ||
| − | |image name= | + | |image name=Iron_electrolytic_and_1cm3_cube.jpg |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|electrons per shell=2, 8, 14, 2 | |electrons per shell=2, 8, 14, 2 | ||
| − | + | |phase= | |
| − | |phase= | ||
|density gpcm3nrt=7.874 | |density gpcm3nrt=7.874 | ||
|density gpcm3mp=6.98 | |density gpcm3mp=6.98 | ||
| Line 50: | Line 42: | ||
|vapor pressure 100 k=3132 | |vapor pressure 100 k=3132 | ||
|vapor pressure comment= | |vapor pressure comment= | ||
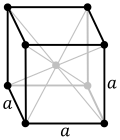
| − | |crystal structure= | + | |crystal structure=body-centered cubic |
| − | + | |crystal structure comment=''a''=286.65 pm | |
| − | + | |crystal structure 2=face-centered cubic | |
| − | + | |crystal structure 2 comment=between 1185–1667 K; ''a''=364.680 pm | |
|electronegativity=1.83 | |electronegativity=1.83 | ||
|number of ionization energies=4 | |number of ionization energies=4 | ||
| − | | | + | |ionization energy 1=762.5 |
| − | | | + | |ionization energy 2=1561.9 |
| − | | | + | |ionization energy 3=2957 |
|atomic radius=126 | |atomic radius=126 | ||
|atomic radius calculated= | |atomic radius calculated= | ||
| − | |covalent radius=132±3 | + | |covalent radius=Low spin: 132±3 pm<br/>High spin: 152±6 |
|Van der Waals radius= | |Van der Waals radius= | ||
|magnetic ordering=[[ferromagnetism|ferromagnetic]] | |magnetic ordering=[[ferromagnetism|ferromagnetic]] | ||
| − | |Curie point=1043 | + | |Curie point K=1043 |
|electrical resistivity= | |electrical resistivity= | ||
| + | |electrical resistivity unit prefix=n | ||
|electrical resistivity at 0= | |electrical resistivity at 0= | ||
| − | |electrical resistivity at 20=96.1 | + | |electrical resistivity at 20=96.1 |
|thermal conductivity=80.4 | |thermal conductivity=80.4 | ||
|thermal conductivity 2= | |thermal conductivity 2= | ||
| Line 75: | Line 68: | ||
|speed of sound= | |speed of sound= | ||
|speed of sound rod at 20= | |speed of sound rod at 20= | ||
| − | |speed of sound rod at r.t.=(electrolytic) | + | |speed of sound rod at r.t.=5120 |
| + | |speed of sound rod at r.t. comment=(electrolytic) | ||
| + | |magnetic susceptibility= | ||
| + | |magnetic susceptibility ref= | ||
|Young's modulus=211 | |Young's modulus=211 | ||
|Shear modulus=82 | |Shear modulus=82 | ||
| Line 82: | Line 78: | ||
|Mohs hardness=4 | |Mohs hardness=4 | ||
|Vickers hardness=608 | |Vickers hardness=608 | ||
| − | |Brinell hardness= | + | |Brinell hardness=200–1180 |
|CAS number=7439-89-6 | |CAS number=7439-89-6 | ||
|isotopes= | |isotopes= | ||
| − | {{ | + | {{infobox element/isotopes stable | mn=54 | sym=Fe | na=5.85% | n=28 |firstlinks=yes}} |
| − | {{ | + | {{infobox element/isotopes decay | mn=55 | sym=Fe | na=[[synthetic radioisotope|syn]] |link=Iron-55 | hl=2.73 y | dm=[[electron capture|ε]] | de=0.231 | link1=manganese-55 | pn=55 | ps=Mn}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=56 | sym=Fe | na=91.75% |
| − | {{ | + | |n=30 | link=Iron-56 |firstlinks=no}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=57 | sym=Fe | na=2.12% |
| − | {{ | + | |n=31 |firstlinks=no}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=58 | sym=Fe | na=0.28% |
| + | |n=32 |firstlinks=no}} | ||
| + | {{infobox element/isotopes decay | mn=59 | sym=Fe | na=syn | hl=44.6 d | dm=[[Beta decay|β<sup>−</sup>]] | de=1.565 | link1=cobalt-59 | pn=59 | ps=Co }} | ||
| + | {{infobox element/isotopes decay | mn=60 | sym=Fe | na=[[trace radioisotope|trace]] | hl=2.6×10<sup>6</sup> y | dm=β<sup>−</sup> | de=3.978 | link1=cobalt-60 | pn=60 | ps=Co }} | ||
|isotopes comment= | |isotopes comment= | ||
| − | }}<noinclude> | + | |discovery date=before [[5000 BC]] |
| − | {{ | + | |QID=Q677 |
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Fe}} | ||
| + | {{Template reference list}} | ||
| + | {{documentation|1=Template:Infobox element/doc}} | ||
| + | </noinclude> | ||
Latest revision as of 14:29, 5 October 2020
[[Category:Template:Pagetype with short description]]
 | |||||||||||||||||||||||||||||||||||||||||
| Iron | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | lustrous metallic with a grayish tinge | ||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Fe) | Template:Val[1] | ||||||||||||||||||||||||||||||||||||||||
| Iron in the periodic table | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 26 | ||||||||||||||||||||||||||||||||||||||||
| Group | group 8 | ||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d6 4s2 | ||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 | ||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||
| Melting point | 1811 K (1538 °C, 2800 °F) | ||||||||||||||||||||||||||||||||||||||||
| Boiling point | 3134 K (2862 °C, 5182 °F) | ||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 7.874 g/cm3 | ||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 6.98 g/cm3 | ||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 13.81 kJ/mol | ||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 340 kJ/mol | ||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.10 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −4, −2, −1, 0, +1,[2] +2, +3, +4, +5,[3] +6, +7[4] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.83 | ||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 126 pm | ||||||||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 132±3 pm High spin: 152±6 pm | ||||||||||||||||||||||||||||||||||||||||
| Spectral lines of iron | |||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) a=286.65 pm | ||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) between 1185–1667 K; a=364.680 pm | ||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5120 m/s (at r.t.) (electrolytic) | ||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 11.8 µm/(m·K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 80.4 W/(m·K) | ||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 96.1 nΩ·m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||
| Curie point | 1043 K | ||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 211 GPa | ||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 82 GPa | ||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 170 GPa | ||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.29 | ||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 4 | ||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 608 MPa | ||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 200–1180 MPa | ||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-89-6 | ||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||
| Discovery | before 5000 BC | ||||||||||||||||||||||||||||||||||||||||
| Main isotopes of iron | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]
| data m.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 1538 | — | — | ||
| K | 1811 | 1811 | 0 | ||
| F | 2800 | 2800 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 1538, K: 1811, F: 2800 | ||||
| comment | |||||
| data b.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 2862 | — | — | ||
| K | 3134 | 3135 | -1 | delta | |
| F | 5182 | 5184 | -2 | delta | Template:Subpage other |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 2862, K: 3134, F: 5182 | ||||
| comment | |||||
| Mn ← |
→ Co | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Ram, R. S.; Bernath, P. F. (2003). "Fourier transform emission spectroscopy of the g4Δ–a4Δ system of FeCl". Journal of Molecular Spectroscopy. 221 (2): 261. Bibcode:2003JMoSp.221..261R. doi:10.1016/S0022-2852(03)00225-X.
- ↑ Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. (1982). "Recent developments in the field of high oxidation states of transition elements in oxides stabilization of six-coordinated Iron(V)". Zeitschrift für anorganische und allgemeine Chemie. 491: 60–66. doi:10.1002/zaac.19824910109.
- ↑ Lu, J.; Jian, J.; Huang, W.; Lin, H.; Li, J; Zhou, M. (2016). "Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−". Physical Chemistry Chemical Physics. 18 (45): 31125–31131. Bibcode:2016PCCP...1831125L. doi:10.1039/C6CP06753K. PMID 27812577.
Lua error: Internal error: The interpreter has terminated with signal "24".