Difference between revisions of "Template:Infobox lawrencium"
Jump to navigation
Jump to search
imported>RaymondSutanto m |
imported>Double sharp |
||
| (60 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Infobox element |
| − | |number=103 | + | |number=103 |
| − | |symbol=Lr | + | |symbol=Lr |
|name=lawrencium | |name=lawrencium | ||
| − | | | + | |abundance= |
| − | |left=[[nobelium]] | + | |abundance in earth's crust= |
| − | |right=[[rutherfordium]] | + | |abundance in oceans= |
| − | |above=[[lutetium|Lu]] | + | |abundance in solar system= |
| − | |below=( | + | |left=[[nobelium]] |
| − | | | + | |right=[[rutherfordium]] |
| − | | | + | |above=[[lutetium|Lu]] |
| − | | | + | |below=(Ups) |
| − | | | + | |period=7 |
| − | | | + | |group=3 |
| − | | | + | |block=d |
| − | | | + | |appearance=silvery ''(predicted)''<ref name="emsley">{{cite book|last=Emsley|first=John|title=Nature's Building Blocks: An A-Z Guide to the Elements|edition=New|year=2011|publisher=Oxford University Press|location=New York, NY|isbn=978-0-19-960563-7|page=278–9}}</ref> |
| − | | | + | |electrons per shell=2, 8, 18, 32, 32, 8, 3 |
| − | |electrons per shell= 2, 8, 18, 32, 32, 8, 3 | + | |phase=solid |
| − | |phase= solid | + | |phase comment=''(predicted)'' |
| − | |phase comment = | + | |density gpcm3nrt=~15.6–16.6 |
| − | | | + | |density gpcm3nrt comment=''(predicted)''<ref name=density>{{cite journal |last=Fournier |first=Jean-Marc |date=1976 |title=Bonding and the electronic structure of the actinide metals |journal=Journal of Physics and Chemistry of Solids |volume=37 |issue=2 |pages=235–244 |doi=10.1016/0022-3697(76)90167-0|bibcode=1976JPCS...37..235F }}</ref><ref name=Penneman>{{cite journal |last=Penneman |first=R. A. |last2=Mann |first2=J. B. |date=1976 |title='Calculation chemistry' of the superheavy elements; comparison with elements of the 7th period |journal=Proceedings of the Moscow Symposium on the Chemistry of Transuranium Elements |pages=257–263 |doi=10.1016/B978-0-08-020638-7.50053-1 }}</ref> |
| − | |number of ionization energies=3 | + | |crystal structure=hexagonal close-packed |
| − | | | + | |crystal structure comment=''(predicted)''<ref name=hcp>{{cite journal|doi=10.1103/PhysRevB.84.113104|title=First-principles calculation of the structural stability of 6d transition metals|year=2011|last1=Östlin|first1=A.|last2=Vitos|first2=L.|journal=Physical Review B|volume=84|issue=11|bibcode=2011PhRvB..84k3104O }}</ref> |
| − | | | + | |electronegativity=1.3 |
| − | | | + | |electronegativity comment=''(predicted)''<ref>{{cite book |last=Brown |first=Geoffrey |date=2012 |title=The Inaccessible Earth: An integrated view to its structure and composition |publisher=Springer Science & Business Media |page=88 |isbn=9789401115162}}</ref> |
| − | |CAS number= 22537-19-5 | + | |melting point K=1900 |
| + | |melting point C=1627 | ||
| + | |melting point F=2961 | ||
| + | |melting point comment=''(predicted)'' | ||
| + | |number of ionization energies=3 | ||
| + | |ionization energy 1=478.6 | ||
| + | |ionization energy 1 ref=<ref>http://cen.acs.org/articles/93/i15/Lawrencium-Ionization-Energy-Measured.html?cq_ck=1428631698138</ref> | ||
| + | |ionization energy 2=1428.0 | ||
| + | |ionization energy 2 comment = ''(predicted)'' | ||
| + | |ionization energy 3=2219.1 | ||
| + | |ionization energy 3 comment = ''(predicted)'' | ||
| + | |CAS number=22537-19-5 | ||
| + | |magnetic susceptibility= | ||
| + | |magnetic susceptibility ref= | ||
|isotopes= | |isotopes= | ||
| − | {{ | + | {{infobox element/isotopes decay2 | mn=254 | sym=Lr | na=syn | hl=13 s |
| − | {{ | + | |dm1=78% α | de1=8.46, 8.41 | link1=mendelevium-250 | pn1=250 | ps1=Md |
| − | {{ | + | |dm2=22% ε | de2= | link2=nobelium-254 | pn2=254 | ps2=No}} |
| − | {{ | + | {{infobox element/isotopes decay | mn=255 | sym=Lr | na=syn | hl=21.5 s | dm=α | de=8.43, 8.37 | link1=mendelevium-251 | pn=251 | ps=Md}} |
| − | {{ | + | {{infobox element/isotopes decay | mn=256 | sym=Lr | na=syn | hl=27 s | dm=α | de=8.62, 8.52, 8.32, ... | link1=mendelevium-252 | pn=252 | ps=Md}} |
| − | {{ | + | {{infobox element/isotopes decay2 | mn=259 | sym=Lr | na=syn | hl=6.2 s | dm1=78% α |
| − | {{ | + | |de1=8.44 | link1=mendelevium-255 | pn1=255 | ps1=Md |
| − | {{ | + | |dm2=22% SF | de2= | pn2= | ps2=}} |
| − | {{ | + | {{infobox element/isotopes decay | mn=260 | sym=Lr | na=syn | hl=2.7 min | dm=[[alpha decay|α]] | de=8.04 | link1=mendelevium-256 | pn=256 | ps=Md}} |
| − | + | {{infobox element/isotopes decay | mn=261 | sym=Lr | na=syn | hl=44 min | dm=SF/ε? | de= | pn= | ps=}} | |
| − | + | {{infobox element/isotopes decay | mn=262 | sym=Lr | na=syn | hl=3.6 h | dm=[[electron capture|ε]] | de= | link1=nobelium-262 | pn=262 | ps=No}} | |
| − | + | {{infobox element isotopes/isotopes decay | mn=264 | sym=Lr | na=syn | hl=3 h<ref>http://flerovlab.jinr.ru/index.php/2020/12/25/she-factory-first-experiment/</ref> | dm=SF | de= | link1= | pn=| ps=}} | |
| − | | | + | {{infobox element/isotopes decay | mn=266 | sym=Lr | na=[[synthetic radioisotope|syn]] | hl=10 h | dm=[[spontaneous fission|SF]] | de= | link1= | pn=| ps=}} |
| − | }} | + | |naming=after [[Ernest Lawrence]] |
| − | + | |discovered by=[[Lawrence Berkeley National Laboratory]] and [[Joint Institute for Nuclear Research]] | |
| + | |discovery date=1961–1971 | ||
| + | |QID=Q1905 | ||
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Lr}} | ||
| + | {{Template reference list}} | ||
| + | {{documentation|1=Template:Infobox element/doc}} | ||
</noinclude> | </noinclude> | ||
Latest revision as of 05:55, 9 February 2021
[[Category:Template:Pagetype with short description]]
| Lawrencium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ləˈrɛnsiəm/ ( | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | [266] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lawrencium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 103 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 7s2 7p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 8, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1900 K (1627 °C, 2961 °F) (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | ~15.6–16.6 g/cm3 (predicted)[2][3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.3 (predicted)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | synthetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
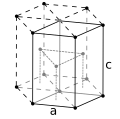
| Crystal structure | hexagonal close-packed (hcp) (predicted)[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 22537-19-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Ernest Lawrence | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Lawrence Berkeley National Laboratory and Joint Institute for Nuclear Research (1961–1971) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of lawrencium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]
| data m.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 1627 | — | — | ||
| K | 1900 | 1900 | 0 | ||
| F | 2961 | 2961 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 1627, K: 1900, F: 2961 | ||||
| comment | (predicted) | ||||
Check temperatures Lr: no input for C, K, F.
| No ← |
→ Rf | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 278–9. ISBN 978-0-19-960563-7.
- ↑ Fournier, Jean-Marc (1976). "Bonding and the electronic structure of the actinide metals". Journal of Physics and Chemistry of Solids. 37 (2): 235–244. Bibcode:1976JPCS...37..235F. doi:10.1016/0022-3697(76)90167-0.
- ↑ Penneman, R. A.; Mann, J. B. (1976). "'Calculation chemistry' of the superheavy elements; comparison with elements of the 7th period". Proceedings of the Moscow Symposium on the Chemistry of Transuranium Elements: 257–263. doi:10.1016/B978-0-08-020638-7.50053-1.
- ↑ Brown, Geoffrey (2012). The Inaccessible Earth: An integrated view to its structure and composition. Springer Science & Business Media. p. 88. ISBN 9789401115162.
- ↑ http://cen.acs.org/articles/93/i15/Lawrencium-Ionization-Energy-Measured.html?cq_ck=1428631698138
- ↑ Östlin, A.; Vitos, L. (2011). "First-principles calculation of the structural stability of 6d transition metals". Physical Review B. 84 (11). Bibcode:2011PhRvB..84k3104O. doi:10.1103/PhysRevB.84.113104.
- ↑ http://flerovlab.jinr.ru/index.php/2020/12/25/she-factory-first-experiment/
Lua error: Internal error: The interpreter has terminated with signal "24".