Difference between revisions of "Template:Infobox titanium"
Jump to navigation
Jump to search
(Added in the Classifaction and family) |
imported>Monkbot m (Task 18a (cosmetic) (manual): eval 1 template: del empty params (2×);) |
||
| (128 intermediate revisions by 74 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{infobox element |
| − | + | |number=22 | |
| − | + | |symbol=Ti | |
| + | |name=titanium | ||
| + | |abundance= | ||
| + | |abundance in earth's crust= | ||
| + | |abundance in oceans= | ||
| + | |abundance in solar system= | ||
| + | |left=[[scandium]] | ||
| + | |right=[[vanadium]] | ||
| + | |above=– | ||
| + | |below=[[zirconium|Zr]] | ||
|group=4 | |group=4 | ||
|period=4 | |period=4 | ||
|block=d | |block=d | ||
| − | |appearance=silvery metallic | + | |appearance=silvery grey-white metallic |
| − | |image name= | + | |image name=Titan-crystal bar.JPG |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|electrons per shell=2, 8, 10, 2 | |electrons per shell=2, 8, 10, 2 | ||
| − | |phase= | + | |phase= |
|density gplstp= | |density gplstp= | ||
|density gpcm3nrt=4.506 | |density gpcm3nrt=4.506 | ||
| Line 39: | Line 43: | ||
|vapor pressure 100 k=3558 | |vapor pressure 100 k=3558 | ||
|vapor pressure comment= | |vapor pressure comment= | ||
| − | |crystal structure=hexagonal | + | |crystal structure=hexagonal close packed |
| − | |||
| − | |||
|electronegativity=1.54 | |electronegativity=1.54 | ||
|number of ionization energies=4 | |number of ionization energies=4 | ||
| − | | | + | |ionization energy 1=658.8 |
| − | | | + | |ionization energy 2=1309.8 |
| − | | | + | |ionization energy 3=2652.5 |
| − | |atomic radius= | + | |atomic radius=147 |
| − | |atomic radius calculated= | + | |atomic radius calculated= |
| − | |covalent radius= | + | |covalent radius=160±8 |
|Van der Waals radius= | |Van der Waals radius= | ||
|magnetic ordering=[[paramagnetic]] | |magnetic ordering=[[paramagnetic]] | ||
| − | |electrical resistivity= | + | |electrical resistivity unit prefix=n |
|electrical resistivity at 0= | |electrical resistivity at 0= | ||
| − | |electrical resistivity at 20= | + | |electrical resistivity at 20=420 |
|thermal conductivity=21.9 | |thermal conductivity=21.9 | ||
|thermal conductivity 2= | |thermal conductivity 2= | ||
| Line 63: | Line 65: | ||
|speed of sound rod at 20= | |speed of sound rod at 20= | ||
|speed of sound rod at r.t.=5090 | |speed of sound rod at r.t.=5090 | ||
| + | |magnetic susceptibility= +153.0·10<sup>−6</sup> | ||
| + | |magnetic susceptibility ref=  (293 K)<ref>{{Cite book|title=CRC, Handbook of Chemistry and Physics|last=Weast|first=Robert|publisher=Chemical Rubber Company Publishing|year=1984|isbn=0-8493-0464-4|location=Boca Raton, Florida|pages=E110}}</ref> | ||
|Young's modulus=116 | |Young's modulus=116 | ||
|Shear modulus=44 | |Shear modulus=44 | ||
|Bulk modulus=110 | |Bulk modulus=110 | ||
|Poisson ratio=0.32 | |Poisson ratio=0.32 | ||
| − | |Mohs hardness=6.0 | + | |Mohs hardness=6.0 |
| − | |Vickers hardness= | + | |Vickers hardness=830–3420 |
| − | |Brinell hardness= | + | |Brinell hardness=716–2770 |
|CAS number=7440-32-6 | |CAS number=7440-32-6 | ||
|isotopes= | |isotopes= | ||
| − | {{ | + | {{infobox element/isotopes decay2 |
| − | dm2=[[gamma | + | |mn=44 | sym=Ti | na=[[synthetic radioisotope|syn]] | hl=63 y |
| − | {{ | + | |dm1=[[electron capture|ε]] | de1=– | link1=scandium-44 | pn1=44 | ps1=Sc |
| − | {{ | + | |dm2=[[gamma radiation|γ]] | de2=0.07[[Delayed nuclear radiation|D]], 0.08D | pn2= | ps2=– }} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=46 | sym=Ti | na=8.25% | n=24 |firstlinks=yes}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=47 | sym=Ti | na=7.44% | n=25 |firstlinks=no}} |
| − | {{ | + | {{Infobox element/isotopes stable | mn=48 | sym=Ti | na=73.72% | n=26 |firstlinks=no}} |
| − | }} | + | {{Infobox element/isotopes stable | mn=49 | sym=Ti | na=5.41% | n=27 |firstlinks=no}} |
| + | {{Infobox element/isotopes stable | mn=50 | sym=Ti | na=5.18% | n=28 |firstlinks=no}} | ||
| + | |discovered by=[[William Gregor]] | ||
| + | |discovery date=1791 | ||
| + | |first isolation by=[[Jöns Jakob Berzelius]] | ||
| + | |first isolation date=1825 | ||
| + | |named by=[[Martin Heinrich Klaproth]] | ||
| + | |named date=1795 | ||
| + | |QID=Q716 | ||
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Ti}} | ||
| + | {{Template reference list}} | ||
| + | {{documentation|1=Template:Infobox element/doc}} | ||
| + | </noinclude> | ||
Latest revision as of 18:08, 16 December 2020
[[Category:Template:Pagetype with short description]]
| Titanium | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /tɪˈteɪniəm, taɪ-/[1] | |||||||||||||||||||||||||||||||||||||
| Appearance | silvery grey-white metallic | |||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Ti) | Template:Val[2] | |||||||||||||||||||||||||||||||||||||
| Titanium in the periodic table | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 22 | |||||||||||||||||||||||||||||||||||||
| Group | group 4 | |||||||||||||||||||||||||||||||||||||
| Period | period 4 | |||||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d2 4s2 | |||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 10, 2 | |||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||
| Melting point | 1941 K (1668 °C, 3034 °F) | |||||||||||||||||||||||||||||||||||||
| Boiling point | 3560 K (3287 °C, 5949 °F) | |||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 4.506 g/cm3 | |||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 4.11 g/cm3 | |||||||||||||||||||||||||||||||||||||
| Heat of fusion | 14.15 kJ/mol | |||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 425 kJ/mol | |||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.060 J/(mol·K) | |||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, −1, 0,[3] +1, +2, +3, +4[4] (an amphoteric oxide) | |||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.54 | |||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 147 pm | |||||||||||||||||||||||||||||||||||||
| Covalent radius | 160±8 pm | |||||||||||||||||||||||||||||||||||||
| Spectral lines of titanium | ||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||
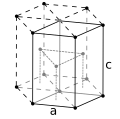
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 5090 m/s (at r.t.) | |||||||||||||||||||||||||||||||||||||
| Thermal expansion | 8.6 µm/(m·K) (at 25 °C) | |||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 21.9 W/(m·K) | |||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 420 nΩ·m (at 20 °C) | |||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||||||||||||||||||||||||
| Magnetic susceptibility | +153.0·10−6 cm3/mol (293 K)[5] | |||||||||||||||||||||||||||||||||||||
| Young's modulus | 116 GPa | |||||||||||||||||||||||||||||||||||||
| Shear modulus | 44 GPa | |||||||||||||||||||||||||||||||||||||
| Bulk modulus | 110 GPa | |||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.32 | |||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.0 | |||||||||||||||||||||||||||||||||||||
| Vickers hardness | 830–3420 MPa | |||||||||||||||||||||||||||||||||||||
| Brinell hardness | 716–2770 MPa | |||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-32-6 | |||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||
| Discovery | William Gregor (1791) | |||||||||||||||||||||||||||||||||||||
| First isolation | Jöns Jakob Berzelius (1825) | |||||||||||||||||||||||||||||||||||||
| Named by | Martin Heinrich Klaproth (1795) | |||||||||||||||||||||||||||||||||||||
| Main isotopes of titanium | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]
| data m.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 1668 | — | — | ||
| K | 1941 | 1941 | 0 | ||
| F | 3034 | 3034 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 1668, K: 1941, F: 3034 | ||||
| comment | |||||
| data b.p. cat | |||||
|---|---|---|---|---|---|
| in | calc from C | diff | report | ref | |
| C | 3287 | — | — | ||
| K | 3560 | 3560 | 0 | ||
| F | 5949 | 5949 | 0 | Template:Subpage other | |
| max precision | 0 | ||||
| WD | Template:Wikidata Script error: No such module "EditAtWikidata". | Template:Wikidata | |||
| input | C: 3287, K: 3560, F: 5949 | ||||
| comment | |||||
| Sc ← |
→ V | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ "titanium - definition of titanium in English | Oxford Dictionaries". Oxford University Press. 2017. Retrieved 2017-03-28.
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G., Jr.; Ellis, John E. (2007). "Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)4(S2CNR2)]−". Chem. Commun. (25): 2639–2641. doi:10.1039/B700808B. PMID 17579764.
- ↑ Andersson, N.; et al. (2003). "Emission spectra of TiH and TiD near 938 nm" (PDF). J. Chem. Phys. 118 (8): 10543. Bibcode:2003JChPh.118.3543A. doi:10.1063/1.1539848.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
Lua error: Internal error: The interpreter has terminated with signal "24".