Difference between revisions of "Template:Did you know nominations/Finafloxacin"
Jump to navigation
Jump to search
imported>Andrew Davidson (review) |
imported>Biochemistry&Love (Reply) |
||
| Line 11: | Line 11: | ||
--> | --> | ||
| + | <div style="float:right; margin-left:0.5em;" id="mp-dyk-img"> | ||
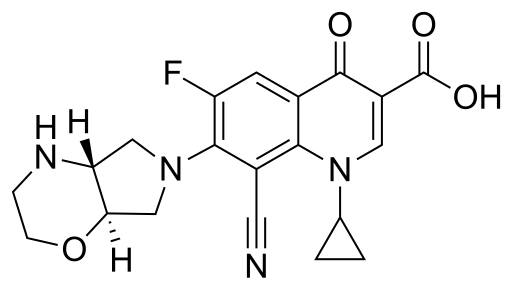
| + | {{main page image|image=Finafloxacin.svg|caption=Finafloxacin's chemical structure in 2D format.|width=120x133}} | ||
| + | </div> | ||
* ... that '''[[Xtoro]]''' likes dropping [[Hydrochloric acid|acid]] and giving [[Quinolone_antibiotic#Mechanism_of_action|bad trips]] to its [[bacteria|enemies]]? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | * ... that '''[[Xtoro]]''' likes dropping [[Hydrochloric acid|acid]] and giving [[Quinolone_antibiotic#Mechanism_of_action|bad trips]] to its [[bacteria|enemies]]? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | ||
** '''ALT1''':... that [[bacteria]] shouldn't drop [[Hydrochloric acid|acid]] while taking '''[[finafloxacin]]'''? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | ** '''ALT1''':... that [[bacteria]] shouldn't drop [[Hydrochloric acid|acid]] while taking '''[[finafloxacin]]'''? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | ||
** '''ALT2''':... that '''[[finafloxacin]]''' is a new [[fluoroquinolone]] antibiotic that works best with [[Hydrochloric acid|acid]]? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | ** '''ALT2''':... that '''[[finafloxacin]]''' is a new [[fluoroquinolone]] antibiotic that works best with [[Hydrochloric acid|acid]]? <small>Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". ''Drugs''. 75: 687–693. doi:10.1007/s40265-015-0384-z.)</small> | ||
| − | :* | + | :* '''ALT3''':... that '''[[finafloxacin]]''' is a new treatment for [[otitis externa|swimmer's ear]]? |
:* ''Comment'': ALT0-1 are more silly. ALT2 is more serious. All are referencing the same thing. I still have <5 DYK credits. | :* ''Comment'': ALT0-1 are more silly. ALT2 is more serious. All are referencing the same thing. I still have <5 DYK credits. | ||
<small>5x expanded by [[User:Biochemistry&Love|Biochemistry&Love]] ([[User talk:Biochemistry&Love|talk]]). Self-nominated at 01:03, 15 August 2017 (UTC).</small> | <small>5x expanded by [[User:Biochemistry&Love|Biochemistry&Love]] ([[User talk:Biochemistry&Love|talk]]). Self-nominated at 01:03, 15 August 2017 (UTC).</small> | ||
| Line 40: | Line 43: | ||
|sign = [[user:Andrew Davidson|Andrew D.]] ([[user talk:Andrew Davidson|talk]]) 17:33, 24 August 2017 (UTC) | |sign = [[user:Andrew Davidson|Andrew D.]] ([[user talk:Andrew Davidson|talk]]) 17:33, 24 August 2017 (UTC) | ||
}} | }} | ||
| − | + | :@[[user:Andrew Davidson|Andrew D.]]: Thank you kindly for your review! I've tried to clean up some of the jargon in the article and wikified some things. Thank you for pointing out the weasel wording; I've specified the referenced source in that sentence. I've added a picture as well, as requested! (: In terms of the interesting portion, I suppose it is a bit forced, haha. I mean, it's a fact that it works best under acidic conditions, but what's disputed is whether that will translate to clinical efficacy dealing with infections in acidic places (e.g. stomach). From a pharmacological stand point, I think the most notable thing about [[finafloxacin]] is its difference from other [[fluoroquinolones]], but perhaps something about swimmer's ear would be accessible to a larger audience. I've added an ALT3. ―[[User:Biochemistry&Love|<span style="letter-spacing:1px"><span style="color:Teal">'''Bio'''</span><span style="color:seagreen">chemistry</span><span style="color:Teal">🙴</span><span style="color:firebrick">❤</span></span>]] 01:37, 25 August 2017 (UTC) | |
}}<!--Please do not write below this line or remove this line. Place comments above this line.--> | }}<!--Please do not write below this line or remove this line. Place comments above this line.--> | ||
Revision as of 01:38, 25 August 2017
| DYK toolbox |
|---|
Finafloxacin
- ... that Xtoro likes dropping acid and giving bad trips to its enemies? Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". Drugs. 75: 687–693. doi:10.1007/s40265-015-0384-z.)
- ALT1:... that bacteria shouldn't drop acid while taking finafloxacin? Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". Drugs. 75: 687–693. doi:10.1007/s40265-015-0384-z.)
- ALT2:... that finafloxacin is a new fluoroquinolone antibiotic that works best with acid? Source: "The compound exhibits optimal efficacy in slightly acidic environments (pH 5.0–6.0), under which other fluoroquinolones lose activity." (McKeage, Kate (2015). "Finafloxacin: First Global Approval". Drugs. 75: 687–693. doi:10.1007/s40265-015-0384-z.)
- ALT3:... that finafloxacin is a new treatment for swimmer's ear?
- Comment: ALT0-1 are more silly. ALT2 is more serious. All are referencing the same thing. I still have <5 DYK credits.
5x expanded by Biochemistry&Love (talk). Self-nominated at 01:03, 15 August 2017 (UTC).
Lua error: expandTemplate: template "y" does not exist.
- @Andrew D.: Thank you kindly for your review! I've tried to clean up some of the jargon in the article and wikified some things. Thank you for pointing out the weasel wording; I've specified the referenced source in that sentence. I've added a picture as well, as requested! (: In terms of the interesting portion, I suppose it is a bit forced, haha. I mean, it's a fact that it works best under acidic conditions, but what's disputed is whether that will translate to clinical efficacy dealing with infections in acidic places (e.g. stomach). From a pharmacological stand point, I think the most notable thing about finafloxacin is its difference from other fluoroquinolones, but perhaps something about swimmer's ear would be accessible to a larger audience. I've added an ALT3. ―Biochemistry🙴❤ 01:37, 25 August 2017 (UTC)