Difference between revisions of "Template:Infobox caesium/sandbox"
Jump to navigation
Jump to search
imported>DePiep m (deprecated parameter |series, now |category (via JWB)) |
imported>Gonnym |
||
| Line 1: | Line 1: | ||
| − | + | {{Infobox element | |
| − | {{ | + | |engvar={{{engvar|}}} |
|number=55 | |number=55 | ||
|symbol=Cs | |symbol=Cs | ||
|name=caesium | |name=caesium | ||
| − | |alt name=cesium | + | |alt name=cesium (US, informal) |
| − | | | + | |abundance= |
| − | | | + | |abundance in earth's crust= |
| + | |abundance in oceans= | ||
| + | |abundance in solar system= | ||
|left=[[xenon]] | |left=[[xenon]] | ||
|right=[[barium]] | |right=[[barium]] | ||
|above=[[rubidium|Rb]] | |above=[[rubidium|Rb]] | ||
|below=[[francium|Fr]] | |below=[[francium|Fr]] | ||
| − | |||
|category comment= | |category comment= | ||
|group=1 | |group=1 | ||
|period=6 | |period=6 | ||
|block=s | |block=s | ||
| − | + | |appearance=pale gold | |
| − | |appearance= | ||
|image name=Cesium.jpg | |image name=Cesium.jpg | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|electrons per shell=2, 8, 18, 18, 8, 1 | |electrons per shell=2, 8, 18, 18, 8, 1 | ||
| − | + | |phase= | |
| − | |phase= | ||
|phase comment= | |phase comment= | ||
|density gplstp= | |density gplstp= | ||
|density gpcm3nrt=1.93 | |density gpcm3nrt=1.93 | ||
|density gpcm3nrt 2= | |density gpcm3nrt 2= | ||
| − | |||
|density gpcm3mp=1.843 | |density gpcm3mp=1.843 | ||
|melting point K=301.7 | |melting point K=301.7 | ||
| Line 63: | Line 51: | ||
|vapor pressure 100 k=940 | |vapor pressure 100 k=940 | ||
|vapor pressure comment= | |vapor pressure comment= | ||
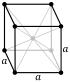
| − | |crystal structure= | + | |crystal structure=bodycentredcubic |
| − | |||
| − | |||
|electronegativity=0.79 | |electronegativity=0.79 | ||
|number of ionization energies=3 | |number of ionization energies=3 | ||
| Line 89: | Line 75: | ||
|speed of sound rod at 20= | |speed of sound rod at 20= | ||
|speed of sound rod at r.t.= | |speed of sound rod at r.t.= | ||
| + | |magnetic susceptibility= | ||
| + | |magnetic susceptibility ref= | ||
|tensile strength= | |tensile strength= | ||
|Young's modulus=1.7 | |Young's modulus=1.7 | ||
| Line 98: | Line 86: | ||
|Brinell hardness=0.14 | |Brinell hardness=0.14 | ||
|CAS number=7440-46-2 | |CAS number=7440-46-2 | ||
| − | |isotopes={{infobox element/isotopes | + | |isotopes={{infobox element/isotopes stable | mn=133 | sym=Cs | na=100% | n=78 |firstlinks=yes }} |
{{infobox element/isotopes decay2 | mn=134 | sym=Cs | {{infobox element/isotopes decay2 | mn=134 | sym=Cs | ||
| − | + | |na=[[synthetic radioisotope|syn]] | hl=2.0648 y | |
| − | + | |dm1=[[electron capture|ε]] | de1=1.229 | link1=xenon-134 | pn1=134 | ps1=Xe | |
| − | + | |dm2=[[beta emission|β<sup>−</sup>]] | de2=2.059 | link2=barium-134 | pn2=134 | ps2=Ba}} | |
{{infobox element/isotopes decay | mn=135 | sym=Cs | {{infobox element/isotopes decay | mn=135 | sym=Cs | ||
| − | + | |na=[[trace radioisotope|trace]] | hl=2.3×10<sup>6</sup> y | |
| − | + | |dm=β<sup>−</sup> | de=0.269 | link1=barium-135 | pn=135 | ps=Ba}} | |
| − | {{infobox element/isotopes decay | mn=137 | sym=Cs | + | {{infobox element/isotopes decay |link=Caesium-137 | mn=137 | sym=Cs |
| − | + | |na=syn | hl=30.17 y<ref name=isotope>{{cite web|title=NIST Radionuclide Half-Life Measurements|url=http://www.nist.gov/pml/data/halflife.cfm| work=[[NIST]]|accessdate=2011-03-13}}</ref> | |
| − | + | |dm=β<sup>−</sup> | de=1.174 |link1=barium-137 | pn=137 | ps=Ba}} | |
| − | |isotopes comment= | + | |isotopes comment= |
| − | |naming=from Latin {{lang|la| | + | |naming=from Latin {{lang|la|caesius}}, sky blue, for its spectral colours |
|discovered by=[[Robert Bunsen]] and [[Gustav Kirchhoff]] | |discovered by=[[Robert Bunsen]] and [[Gustav Kirchhoff]] | ||
|discovery date=1860 | |discovery date=1860 | ||
|first isolation by=[[Carl Setterberg]] | |first isolation by=[[Carl Setterberg]] | ||
|first isolation date=1882 | |first isolation date=1882 | ||
| − | }}<noinclude> | + | |QID=Q1108 |
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Cs}} | ||
{{Template reference list}} | {{Template reference list}} | ||
{{documentation|1=Template:Infobox element/doc}} | {{documentation|1=Template:Infobox element/doc}} | ||
</noinclude> | </noinclude> | ||
Latest revision as of 11:15, 1 November 2020
[[Category:Template:Pagetype with short description]]
 | ||||||||||||||||||||||||||||
| Caesium | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈsiːziəm/ | |||||||||||||||||||||||||||
| Alternative name | cesium (US, informal) | |||||||||||||||||||||||||||
| Appearance | pale gold | |||||||||||||||||||||||||||
| Standard atomic weight Ar, std(Cs) | Template:Val[1] | |||||||||||||||||||||||||||
| Caesium in the periodic table | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Atomic number (Z) | 55 | |||||||||||||||||||||||||||
| Group | group 1: H and alkali metals | |||||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||||
| Block | s-block | |||||||||||||||||||||||||||
| Electron configuration | [Xe] 6s1 | |||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 8, 1 | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||
| Melting point | 301.7 K (28.5 °C, 83.3 °F) | |||||||||||||||||||||||||||
| Boiling point | 944 K (671 °C, 1240 °F) | |||||||||||||||||||||||||||
| Density (near r.t.) | 1.93 g/cm3 | |||||||||||||||||||||||||||
| when liquid (at m.p.) | 1.843 g/cm3 | |||||||||||||||||||||||||||
| Critical point | 1938 K, 9.4 MPa[2] | |||||||||||||||||||||||||||
| Heat of fusion | 2.09 kJ/mol | |||||||||||||||||||||||||||
| Heat of vaporization | 63.9 kJ/mol | |||||||||||||||||||||||||||
| Molar heat capacity | 32.210 J/(mol·K) | |||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Oxidation states | −1, +1[3] (a strongly basic oxide) | |||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.79 | |||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||
| Atomic radius | empirical: 265 pm | |||||||||||||||||||||||||||
| Covalent radius | 244±11 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 343 pm | |||||||||||||||||||||||||||
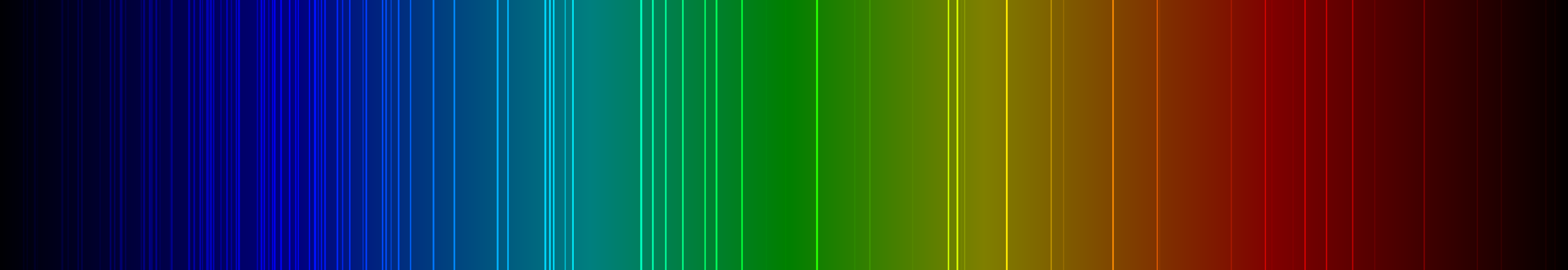
| Spectral lines of caesium | ||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) | |||||||||||||||||||||||||||
| Thermal expansion | 97 µm/(m·K) (at 25 °C) | |||||||||||||||||||||||||||
| Thermal conductivity | 35.9 W/(m·K) | |||||||||||||||||||||||||||
| Electrical resistivity | 205 nΩ·m (at 20 °C) | |||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[4] | |||||||||||||||||||||||||||
| Young's modulus | 1.7 GPa | |||||||||||||||||||||||||||
| Bulk modulus | 1.6 GPa | |||||||||||||||||||||||||||
| Mohs hardness | 0.2 | |||||||||||||||||||||||||||
| Brinell hardness | 0.14 MPa | |||||||||||||||||||||||||||
| CAS Number | 7440-46-2 | |||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||
| Naming | from Latin caesius, sky blue, for its spectral colours | |||||||||||||||||||||||||||
| Discovery | Robert Bunsen and Gustav Kirchhoff (1860) | |||||||||||||||||||||||||||
| First isolation | Carl Setterberg (1882) | |||||||||||||||||||||||||||
| Main isotopes of caesium | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Xe ← |
→ Ba | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Template:RubberBible92nd
- ↑ Dye, J. L. (1979). "Compounds of Alkali Metal Anions". Angewandte Chemie International Edition. 18 (8): 587–598. doi:10.1002/anie.197905871.
- ↑ "Magnetic susceptibility of the elements and inorganic compounds". Handbook of Chemistry and Physics (PDF) (87th ed.). CRC press. ISBN 0-8493-0487-3. Retrieved 2010-09-26.
- ↑ "NIST Radionuclide Half-Life Measurements". NIST. Retrieved 2011-03-13.
Lua error: Internal error: The interpreter has terminated with signal "24".