Difference between revisions of "Template:Infobox lutetium/sandbox"
Jump to navigation
Jump to search
imported>Materialscientist m (Reverted edits by 103.240.125.29 (talk) to last version by DePiep) |
imported>Jonesey95 (sync with live template) |
||
| Line 1: | Line 1: | ||
| − | {{infobox element | + | {{infobox element |
|number=71 | |number=71 | ||
|symbol=Lu | |symbol=Lu | ||
|name=lutetium | |name=lutetium | ||
| − | | | + | |abundance= |
| − | | | + | |abundance in earth's crust= |
| + | |abundance in oceans= | ||
| + | |abundance in solar system= | ||
|left=[[ytterbium]] | |left=[[ytterbium]] | ||
|right=[[hafnium]] | |right=[[hafnium]] | ||
| − | |above= | + | |above=– |
|below=[[lawrencium|Lr]] | |below=[[lawrencium|Lr]] | ||
|category=lanthanide | |category=lanthanide | ||
|category comment=sometimes considered a [[transition metal]] | |category comment=sometimes considered a [[transition metal]] | ||
|period=6 | |period=6 | ||
| − | |block= | + | |block=f |
| − | |group= | + | |group=3 |
|image name=Lutetium_sublimed_dendritic_and_1cm3_cube.jpg | |image name=Lutetium_sublimed_dendritic_and_1cm3_cube.jpg | ||
|appearance=silvery white | |appearance=silvery white | ||
| − | + | |electron configuration=[[[xenon|Xe]]] 4f<sup>14</sup> 5d<sup>1</sup> 6s<sup>2</sup> | |
| − | |||
| − | |electron configuration=[[[xenon|Xe]]] | ||
|electrons per shell=2, 8, 18, 32, 9, 2 | |electrons per shell=2, 8, 18, 32, 9, 2 | ||
|phase=solid | |phase=solid | ||
| Line 40: | Line 40: | ||
|vapor pressure comment= | |vapor pressure comment= | ||
|crystal structure=hexagonal close packed | |crystal structure=hexagonal close packed | ||
| − | |oxidation states=3, 2, 1 | + | |oxidation states='''3''', 2, 1 |
|oxidation states comment=(a weakly [[base (chemistry)|basic]] oxide) | |oxidation states comment=(a weakly [[base (chemistry)|basic]] oxide) | ||
|electronegativity=1.27 | |electronegativity=1.27 | ||
| Line 50: | Line 50: | ||
|covalent radius=187±8 | |covalent radius=187±8 | ||
|magnetic ordering=[[paramagnetic]] | |magnetic ordering=[[paramagnetic]] | ||
| − | |magnetic ordering ref=<ref name=magnet>{{cite book | + | |magnetic ordering ref=<ref name=magnet>{{cite book |url=https://web.archive.org/web/20110303222309/http://www-d0.fnal.gov/hardware/cal/lvps_info/engineering/elementmagn.pdf|chapter=Magnetic susceptibility of the elements and inorganic compounds| editor = Lide, D. R. | title = CRC Handbook of Chemistry and Physics | edition = 86th | location = Boca Raton (FL) | publisher = CRC Press | year = 2005 | isbn = 0-8493-0486-5 }} |
| − | |||
</ref> | </ref> | ||
|electrical resistivity unit prefix=n | |electrical resistivity unit prefix=n | ||
| Line 59: | Line 58: | ||
|thermal expansion=poly: 9.9 | |thermal expansion=poly: 9.9 | ||
|thermal expansion comment=(at {{abbr|r.t.|room temperature}}) | |thermal expansion comment=(at {{abbr|r.t.|room temperature}}) | ||
| + | |magnetic susceptibility= | ||
| + | |magnetic susceptibility ref= | ||
|Young's modulus=68.6 | |Young's modulus=68.6 | ||
|Shear modulus=27.2 | |Shear modulus=27.2 | ||
|Bulk modulus=47.6 | |Bulk modulus=47.6 | ||
|Poisson ratio=0.261 | |Poisson ratio=0.261 | ||
| − | |Vickers hardness= | + | |Vickers hardness=755–1160 |
| − | |Brinell hardness= | + | |Brinell hardness=890–1300 |
|CAS number=7439-94-3 | |CAS number=7439-94-3 | ||
|isotopes= | |isotopes= | ||
{{infobox element/isotopes decay | mn=173 | sym=Lu | {{infobox element/isotopes decay | mn=173 | sym=Lu | ||
| − | + | |na=[[synthetic radioisotope|syn]] | hl=1.37 y | |
| − | + | |dm=[[electron capture|ε]] | de=0.671 | link1=ytterbium-173 | pn=173 | ps=Yb}} | |
{{infobox element/isotopes decay | mn=174 | sym=Lu | {{infobox element/isotopes decay | mn=174 | sym=Lu | ||
| − | + | |na=syn | hl=3.31 y | |
| − | + | |dm=ε | de=1.374 | link1=ytterbium-174 | pn=174 | ps=Yb}} | |
| − | {{infobox element/isotopes | + | {{infobox element/isotopes stable | mn=175 | sym=Lu | na=97.401% | n=104 |firstlinks=yes}} |
{{infobox element/isotopes decay | mn=176 | sym=Lu | {{infobox element/isotopes decay | mn=176 | sym=Lu | ||
| − | + | |na=2.599% | hl=3.78×10<sup>10</sup> y | |
| − | + | |dm=[[beta emission|β<sup>−</sup>]] | de=1.193 | link1=hafnium-176 | pn=176 | ps=Hf}} | |
| − | |isotopes comment= | + | |isotopes comment= |
| − | |naming=after | + | |naming=after {{lang|la|[[Lutetia]]}}, Latin for: Paris, in the Roman era |
| − | |discovered by=[[ | + | |discovered by=[[Carl Auer von Welsbach]] and [[Georges Urbain]] |
|discovery date=1906 | |discovery date=1906 | ||
|first isolation by=Carl Auer von Welsbach | |first isolation by=Carl Auer von Welsbach | ||
|first isolation date=1906 | |first isolation date=1906 | ||
| − | }}<noinclude> | + | |named by=Georges Urbain |
| + | |named date=1906 | ||
| + | |QID=Q1857 | ||
| + | }}<!-- | ||
| + | |||
| + | --><noinclude> | ||
| + | {{Infobox element/element navigation|symbol=Lu}} | ||
{{Template reference list}} | {{Template reference list}} | ||
{{documentation|1=Template:Infobox element/doc}} | {{documentation|1=Template:Infobox element/doc}} | ||
</noinclude> | </noinclude> | ||
Latest revision as of 19:48, 22 May 2019
[[Category:Template:Pagetype with short description]]
 | ||||||||||||||||||||||||||
| Lutetium | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ljuːˈtiːʃiəm/ | |||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||
| Standard atomic weight Ar, std(Lu) | Template:Val[1] | |||||||||||||||||||||||||
| Lutetium in the periodic table | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Atomic number (Z) | 71 | |||||||||||||||||||||||||
| Group | group 3 | |||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||
| Element categories | , sometimes considered a transition metal | |||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d1 6s2 | |||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 9, 2 | |||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||
| Melting point | 1925 K (1652 °C, 3006 °F) | |||||||||||||||||||||||||
| Boiling point | 3675 K (3402 °C, 6156 °F) | |||||||||||||||||||||||||
| Density (near r.t.) | 9.841 g/cm3 | |||||||||||||||||||||||||
| when liquid (at m.p.) | 9.3 g/cm3 | |||||||||||||||||||||||||
| Heat of fusion | ca. 22 kJ/mol | |||||||||||||||||||||||||
| Heat of vaporization | 414 kJ/mol | |||||||||||||||||||||||||
| Molar heat capacity | 26.86 J/(mol·K) | |||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||
| Oxidation states | 0,[2] +1, +2, +3 (a weakly basic oxide) | |||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.27 | |||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||
| Atomic radius | empirical: 174 pm | |||||||||||||||||||||||||
| Covalent radius | 187±8 pm | |||||||||||||||||||||||||
| Spectral lines of lutetium | ||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||
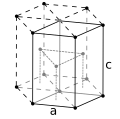
| Crystal structure | hexagonal close-packed (hcp) | |||||||||||||||||||||||||
| Thermal expansion | poly: 9.9 µm/(m·K) (at r.t.) | |||||||||||||||||||||||||
| Thermal conductivity | 16.4 W/(m·K) | |||||||||||||||||||||||||
| Electrical resistivity | poly: 582 nΩ·m (at r.t.) | |||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[3] | |||||||||||||||||||||||||
| Young's modulus | 68.6 GPa | |||||||||||||||||||||||||
| Shear modulus | 27.2 GPa | |||||||||||||||||||||||||
| Bulk modulus | 47.6 GPa | |||||||||||||||||||||||||
| Poisson ratio | 0.261 | |||||||||||||||||||||||||
| Vickers hardness | 755–1160 MPa | |||||||||||||||||||||||||
| Brinell hardness | 890–1300 MPa | |||||||||||||||||||||||||
| CAS Number | 7439-94-3 | |||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||
| Naming | after Lutetia, Latin for: Paris, in the Roman era | |||||||||||||||||||||||||
| Discovery | Carl Auer von Welsbach and Georges Urbain (1906) | |||||||||||||||||||||||||
| First isolation | Carl Auer von Welsbach (1906) | |||||||||||||||||||||||||
| Named by | Georges Urbain (1906) | |||||||||||||||||||||||||
| Main isotopes of lutetium | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Yb ← |
→ Hf | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Yttrium and all lanthanides except Ce and Pm have been observed in the oxidation state 0 in bis(1,3,5-tri-t-butylbenzene) complexes, see Cloke, F. Geoffrey N. (1993). "Zero Oxidation State Compounds of Scandium, Yttrium, and the Lanthanides". Chem. Soc. Rev. 22: 17–24. doi:10.1039/CS9932200017. and Arnold, Polly L.; Petrukhina, Marina A.; Bochenkov, Vladimir E.; Shabatina, Tatyana I.; Zagorskii, Vyacheslav V.; Cloke (2003-12-15). "Arene complexation of Sm, Eu, Tm and Yb atoms: a variable temperature spectroscopic investigation". Journal of Organometallic Chemistry. 688 (1–2): 49–55. doi:10.1016/j.jorganchem.2003.08.028.
- ↑ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
Lua error: Internal error: The interpreter has terminated with signal "24".