Template:Infobox argon/sandbox
{{Infobox
| headerstyle = background:#c0ffff
| labelstyle = padding-right:0.75em;
| datastyle =
| belowstyle = background:#c0ffff
| title = Argon, 18Ar
| image1 = 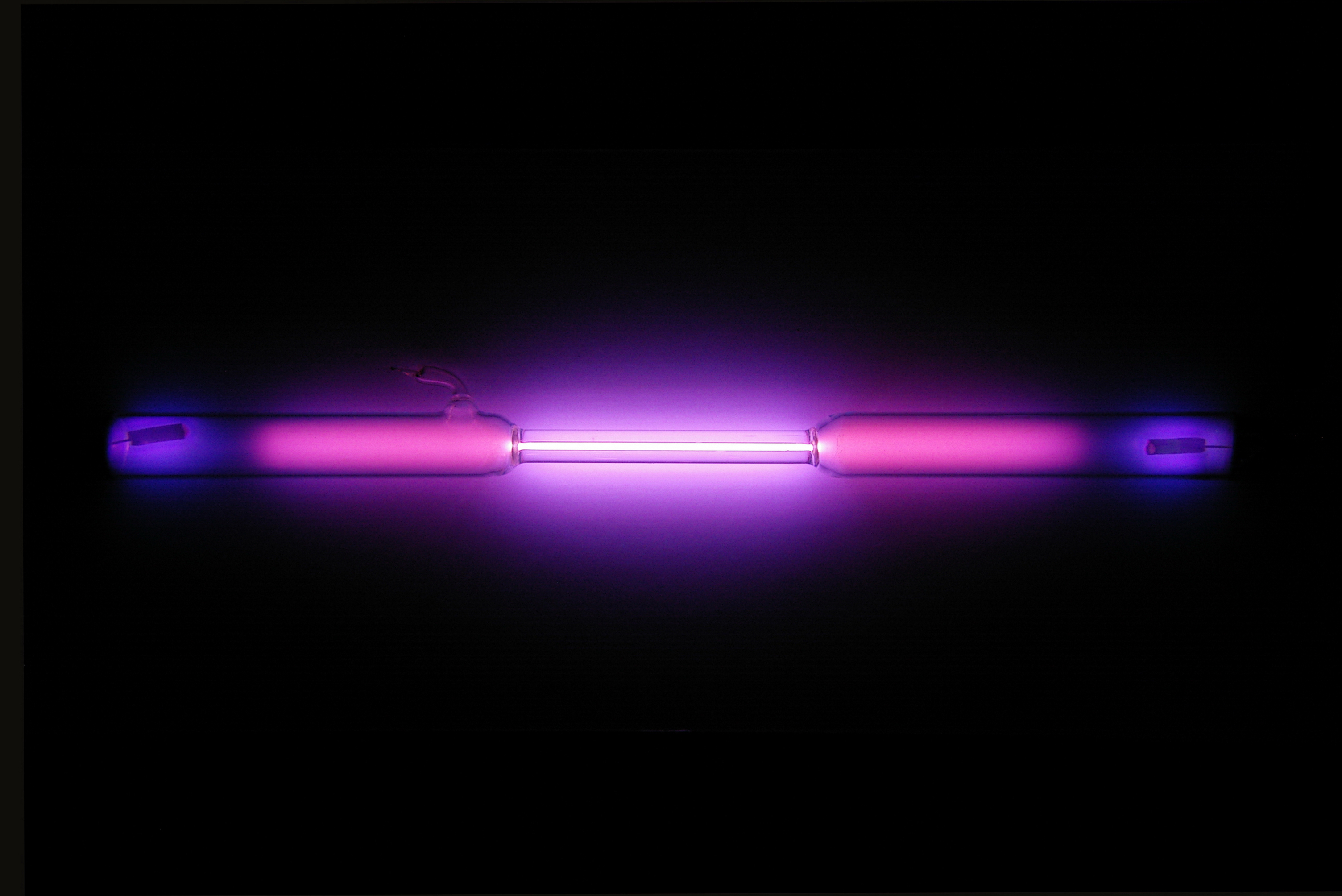 | caption1 =
| image2 =
| caption1 =
| image2 = ![]() | caption2 = Spectral lines of argon[1]| header10 = General properties
| label11 = Pronunciation
| data11 = /ˈɑːrɡɒn/
| caption2 = Spectral lines of argon[1]| header10 = General properties
| label11 = Pronunciation
| data11 = /ˈɑːrɡɒn/
Template:Respell
| label12 = Proposed formal name
| data12 =
| label13 = Alternative name
| data13 =
| label14 = Allotropes
| data14 =
| label15 = Appearance
| data15 = colorless gas exhibiting a lilac/violet glow when placed in an electric field
| label16 = Standard atomic weight (Ar) | data16 = | label17 = Mass number | data17 = | label18 = Abundance | data18 = | data19 = | header20 = Argon in the periodic table | data22 =
| ||||
| label23 = Atomic number (Z) | data23 = 18
| label24 = Group, period | data24 = group 18 (noble gases), period 3 | label25 = Block | data25 = p-block
| label26 = Element category | data26 = noble gas
| label31 = Electron configuration | data31 = [Ne] 3s2 3p6
| label32 =
| data32 = 2, 8, 8
|header40 = Physical properties | data41 = | data42 =
References
- ↑ hello spectral world-old
- ↑ hello spectral world-new
- ↑ Standard Atomic Weights 2013. Commission on Isotopic Abundances and Atomic Weights
- ↑ 4.0 4.1 Template:RubberBible92nd
- ↑ Shuen-Chen Hwang, Robert D. Lein, Daniel A. Morgan (2005). "Noble Gases". Kirk Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01.
- ↑ Magnetic susceptibility of the elements and inorganic compounds, in Template:RubberBible86th
Lua error: Internal error: The interpreter has terminated with signal "24".