Revision as of 22:26, 3 January 2010 by imported>Materialscientist
[[Category:Template:Pagetype with short description]]
Iron, 26 Pure iron chips with a high purity iron cube Iron Appearance lustrous metallic with a grayish tinge Standard atomic weight A r, std (Fe) Template:Val [1] Iron in the periodic table
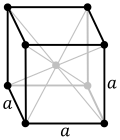
Atomic number (Z ) 26 Group group 8 Period period 4 Block d-block Electron configuration [Ar ] 3d6 4s2 Electrons per shell 2, 8, 14, 2 Physical properties Phase at STP solid Melting point 1811 K (1538 °C, 2800 °F) Boiling point 3134 K (2862 °C, 5182 °F) Density (near r.t. ) 7.874 g/cm3 when liquid (at m.p. ) 6.98 g/cm3 Heat of fusion 13.81 kJ/mol Heat of vaporization 340 kJ/mol Molar heat capacity 25.10 J/(mol·K) Vapor pressure
P (Pa)
1
10
100
1 k
10 k
100 k
at T (K)
1728
1890
2091
2346
2679
3132
Atomic properties Oxidation states −4, −2, −1, 0, +1,[2] +2 +3 [3] +6 [4] amphoteric oxide) Electronegativity Pauling scale: 1.83 Ionization energies Atomic radius empirical: 126 pm Covalent radius 132±3 (low spin), 152±6 (high spin) pm Spectral lines of ironOther properties Natural occurrence primordial Crystal structure body-centered cubic (bcc) Speed of sound thin rod (electrolytic)r.t. ) Thermal expansion 11.8 µm/(m·K) (at 25 °C) Thermal conductivity 80.4 W/(m·K) Electrical resistivity 96.1 n Ω·m (at 20 °C) Magnetic ordering ferromagnetic Young's modulus 211 GPa Shear modulus 82 GPa Bulk modulus 170 GPa Poisson ratio 0.29 Mohs hardness 4 Vickers hardness 608 MPa Brinell hardness 490 MPa CAS Number 7439-89-6 Main isotopes of iron
Category: Iron references
[[Category:Infobox templates|Template:Remove first word ]]
Template:Templaterefsection
↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)" . Pure and Applied Chemistry 88 (3): 265–91. doi :10.1515/pac-2015-0305 ↑ Ram, R. S.; Bernath, P. F. (2003). "Fourier transform emission spectroscopy of the g4 Δ–a4 Δ system of FeCl". Journal of Molecular Spectroscopy . 221 (2): 261. Bibcode :2003JMoSp.221..261R . doi :10.1016/S0022-2852(03)00225-X . ↑ Demazeau, G.; Buffat, B.; Pouchard, M.; Hagenmuller, P. (1982). "Recent developments in the field of high oxidation states of transition elements in oxides stabilization of six-coordinated Iron(V)". Zeitschrift für anorganische und allgemeine Chemie . 491 : 60–66. doi :10.1002/zaac.19824910109 . ↑ Lu, J.; Jian, J.; Huang, W.; Lin, H.; Li, J; Zhou, M. (2016). "Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4 − ". Physical Chemistry Chemical Physics . 18 (45): 31125–31131. Bibcode :2016PCCP...1831125L . doi :10.1039/C6CP06753K . PMID 27812577 .