Revision as of 18:08, 16 December 2020 by imported>Monkbot
[[Category:Template:Pagetype with short description]]
Titanium, 22Ti |
| Titanium |
|---|
| Pronunciation | [1] (Template:Respell) |
|---|
| Appearance | silvery grey-white metallic |
|---|
| Standard atomic weight Ar, std(Ti) | Template:Val[2] |
|---|
| Titanium in the periodic table |
|---|
|
|
| Atomic number (Z) | 22 |
|---|
| Group | group 4 |
|---|
| Period | period 4 |
|---|
| Block | d-block |
|---|
| Electron configuration | [Ar] 3d2 4s2 |
|---|
| Electrons per shell | 2, 8, 10, 2 |
|---|
| Physical properties |
|---|
| Phase at STP | solid |
|---|
| Melting point | 1941 K (1668 °C, 3034 °F) |
|---|
| Boiling point | 3560 K (3287 °C, 5949 °F) |
|---|
| Density (near r.t.) | 4.506 g/cm3 |
|---|
| when liquid (at m.p.) | 4.11 g/cm3 |
|---|
| Heat of fusion | 14.15 kJ/mol |
|---|
| Heat of vaporization | 425 kJ/mol |
|---|
| Molar heat capacity | 25.060 J/(mol·K) |
|---|
Vapor pressure
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1982
|
2171
|
(2403)
|
2692
|
3064
|
3558
|
|
| Atomic properties |
|---|
| Oxidation states | −2, −1, 0,[3] +1, +2, +3, +4[4] (an amphoteric oxide) |
|---|
| Electronegativity | Pauling scale: 1.54 |
|---|
| Ionization energies | - 1st: 658.8 kJ/mol
- 2nd: 1309.8 kJ/mol
- 3rd: 2652.5 kJ/mol
- (more)
|
|---|
| Atomic radius | empirical: 147 pm |
|---|
| Covalent radius | 160±8 pm |
|---|
| Spectral lines of titanium |
| Other properties |
|---|
| Natural occurrence | primordial |
|---|
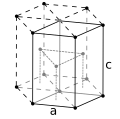
| Crystal structure | hexagonal close-packed (hcp) |
|---|
| Speed of sound thin rod | 5090 m/s (at r.t.) |
|---|
| Thermal expansion | 8.6 µm/(m·K) (at 25 °C) |
|---|
| Thermal conductivity | 21.9 W/(m·K) |
|---|
| Electrical resistivity | 420 nΩ·m (at 20 °C) |
|---|
| Magnetic ordering | paramagnetic |
|---|
| Magnetic susceptibility | +153.0·10−6 cm3/mol (293 K)[5] |
|---|
| Young's modulus | 116 GPa |
|---|
| Shear modulus | 44 GPa |
|---|
| Bulk modulus | 110 GPa |
|---|
| Poisson ratio | 0.32 |
|---|
| Mohs hardness | 6.0 |
|---|
| Vickers hardness | 830–3420 MPa |
|---|
| Brinell hardness | 716–2770 MPa |
|---|
| CAS Number | 7440-32-6 |
|---|
| History |
|---|
| Discovery | William Gregor (1791) |
|---|
| First isolation | Jöns Jakob Berzelius (1825) |
|---|
| Named by | Martin Heinrich Klaproth (1795) |
|---|
| Main isotopes of titanium |
|---|
|
|
 Category: Titanium Category: Titanium
| references |
[[Category:Infobox templates|Template:Remove first word]]
References
These references will appear in the article, but this list appears only on this page.
- ↑ "titanium - definition of titanium in English | Oxford Dictionaries". Oxford University Press. 2017. Retrieved 2017-03-28.
- ↑ Meija, Juris; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- ↑ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G., Jr.; Ellis, John E. (2007). "Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)4(S2CNR2)]−". Chem. Commun. (25): 2639–2641. doi:10.1039/B700808B. PMID 17579764.
- ↑ Andersson, N.; et al. (2003). "Emission spectra of TiH and TiD near 938 nm" (PDF). J. Chem. Phys. 118 (8): 10543. Bibcode:2003JChPh.118.3543A. doi:10.1063/1.1539848.
- ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
Lua error: Internal error: The interpreter has terminated with signal "24".