Template:Antigonadotropic effects of estradiol
- Antigonadotropic effects of estradiol
Testosterone levels in relation to estradiol levels (and corresponding estradiol dosages) during therapy with oral estradiol alone or in combination with an antiandrogen in transgender women.[2] Dashed purple line is upper limit for female/castrate range (~50 ng/dL) and dashed grey line is testosterone level in a comparison group of post-operative transgender women (21.7 pg/mL).[2]
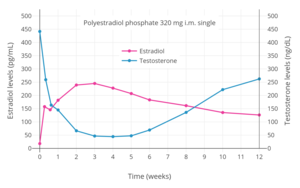
Estradiol and testosterone levels over the course of 12 weeks following a single intramuscular injection of 320 mg polyestradiol phosphate in men with prostate cancer.[3]
Estradiol and testosterone levels with polyestradiol phosphate 160, 240, or 320 mg once every 4 weeks by intramuscular injection in men with prostate cancer.[4]
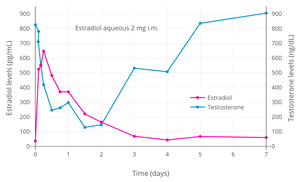
Estradiol, testosterone, and prolactin levels with 100 mg/month estradiol undecylate by intramuscular injection in men with prostate cancer.[8]
See also
References
- ↑ 1.0 1.1 Jones TM, Fang VS, Landau RL, Rosenfield R (December 1978). "Direct inhibition of Leydig cell function by estradiol". J. Clin. Endocrinol. Metab. 47 (6): 1368–73. doi:10.1210/jcem-47-6-1368. PMID 122429.
- ↑ 2.0 2.1 Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- ↑ Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (May 1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- ↑ Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384.
- ↑ Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- ↑ Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani EN, Dearnaley D, Parmar M, Abel PD (August 2008). "Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer". BJU Int. 102 (4): 442–5. doi:10.1111/j.1464-410X.2008.07583.x. PMC 2564109. PMID 18422771.
Available information suggests that for preparations delivering 100 µg/day of oestradiol transdermally (including the Progynova [TS forte] patches used in the original pilot study [5]) [...]
- ↑ Ockrim J, Lalani E, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- ↑ Jacobi, G.H.; Altwein, J.E. (1979). "Bromocriptin als Palliativtherapie beim fortgeschrittenen Prostatakarzinom:Experimentelles und klinisches Profil eines Medikamentes" [Bromocriptine as Palliative Therapy in Advanced Prostate Cancer: Experimental and Clinical Profile of a Drugjournal=Urologia Internationalis]. Urologia Internationalis. 34 (4): 266–290. doi:10.1159/000280272. PMID 89747.