Template:Infobox darmstadtium
Jump to navigation
Jump to search
[[Category:Template:Pagetype with short description]]
| Darmstadtium | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /dɑːrmˈstætiəm, -ˈʃtæt-/ ( | |||||||||||||||||||
| Mass number | [281] | |||||||||||||||||||
| Darmstadtium in the periodic table | ||||||||||||||||||||
| ||||||||||||||||||||
| Atomic number (Z) | 110 | |||||||||||||||||||
| Group | group 10 | |||||||||||||||||||
| Period | period 7 | |||||||||||||||||||
| Block | d-block | |||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d8 7s2 (predicted)[3] | |||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 16, 2 (predicted)[3] | |||||||||||||||||||
| Physical properties | ||||||||||||||||||||
| Phase at STP | solid (predicted)[4] | |||||||||||||||||||
| Density (near r.t.) | 34.8 g/cm3 (predicted)[3] | |||||||||||||||||||
| Atomic properties | ||||||||||||||||||||
| Oxidation states | (0), (+2), (+4), (+6), (+8) (predicted)[3][5] | |||||||||||||||||||
| Ionization energies | ||||||||||||||||||||
| Atomic radius | empirical: 132 pm (predicted)[3][5] | |||||||||||||||||||
| Covalent radius | 128 pm (estimated)[6] | |||||||||||||||||||
| Other properties | ||||||||||||||||||||
| Natural occurrence | synthetic | |||||||||||||||||||
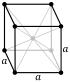
| Crystal structure | body-centered cubic (bcc) (predicted)[4] | |||||||||||||||||||
| CAS Number | 54083-77-1 | |||||||||||||||||||
| History | ||||||||||||||||||||
| Naming | after Darmstadt, Germany, where it was discovered | |||||||||||||||||||
| Discovery | Gesellschaft für Schwerionenforschung (1994) | |||||||||||||||||||
| Main isotopes of darmstadtium | ||||||||||||||||||||
| ||||||||||||||||||||
[[Category:Infobox templates|Template:Remove first word]]Check temperatures Ds: no input for C, K, F.
Check temperatures Ds: no input for C, K, F.
| Mt ← |
→ Rg | |
| ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Top image (caption, alt) | |
| Pronunciation | |
| Category (enwiki) | |
| Standard atomic weight | |
| most stable isotope | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Term symbol * (cmt, ref) | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2019-02-03) See also {{Infobox element/symbol-to--navbox}} | |
References
- ↑ "Darmstadtium". Periodic Table of Videos. The University of Nottingham. Retrieved 19 October 2012.
- ↑ "darmstadtium". Lexico UK Dictionary. Oxford University Press. Retrieved 1 September 2019.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands. ISBN 978-1-4020-3555-5.
- ↑ 4.0 4.1 Östlin, A.; Vitos, L. (2011). "First-principles calculation of the structural stability of 6d transition metals". Physical Review B. 84 (11). Bibcode:2011PhRvB..84k3104O. doi:10.1103/PhysRevB.84.113104.
- ↑ 5.0 5.1 Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013.
- ↑ Chemical Data. Darmstadtium - Ds, Royal Chemical Society
Lua error: Internal error: The interpreter has terminated with signal "24".